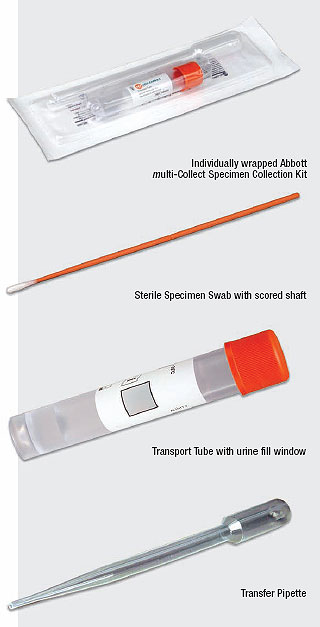
Each multi-Collect Specimen Collection Kit contains:
- Individually packaged sterile specimen collection swab
- Transfer pipette for the collection of urine
- Transport tube with pierceable cap, which contains specimen transport buffer to stabilize DNA until sample preparation
Validated Sample Types for RealTime CT/NG Include:
Female
- Endocervical swab
- Vaginal swab
- Urine
Male
Clinician Benefits
- No additional cleaning steps
- No addition of sample stabilizers
- No stocking of gender-specific collection kits
Laboratorian Benefits
- Pierceable caps
- Pierceable caps eliminate the need to uncap each tube, which:
- Improves lab workflow
- Minimizes cross contamination
- Reduces potential for repetitive motion injuries
- No swab transfer or manual expression of swabs
- No sample centrifugation or vortexing
- No manual pipetting or addition of lysis buffer
Indications and Limitations of Use
Intended Use
The Abbott multi-Collect Specimen Collection Kit is intended for the collection and transportation of male and female, swab and urine specimens for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae per instructions provided. Refer to the specimen collection procedure in the package insert for specimen collection instructions for specific sample types. Self-collected vaginal swab specimens are an option for screening women when a pelvic exam is not otherwise indicated. The Abbott multi-Collect Specimen Collection Kit is not intended for home use.
Warnings and Precautions
- Do not use the Abbott multi-Collect Specimen Collection Kit if the package is damaged, the seal is broken or if buffer has leaked from the tube. Discard unused, damaged, or leaking kits in accordance with local, state, and federal regulations.
- Do not use the Abbott multi-Collect Specimen Collection Kit beyond its expiration date.
- Optimal performance of the Abbott RealTime CT/NG assay requires adequate specimen collection and handling. Ensure the outside of the transport tube and cap are not contaminated.
- Use only the orange shaft swab provided in the Abbott multi-Collect Specimen Collection Kit for collecting swab specimens. The swab must remain in the transport tube after specimen collection. Do not place multiple swabs or a combination of swab and urine in the transport tube.
- Add urine to the transport tube until the liquid level falls within the fill window on the tube label or else a new specimen should be collected.
- The plastic transfer pipette provided in the multi-Collect Specimen Collection Kit is not sterile.
- The presence of blood, mucus, some spermicidal agents, feminine powder sprays, and treatments for vaginal conditions such as yeast infection may interfere with nucleic acid test (NAT) based assays. The effects of other factors such as vaginal discharge, use of tampons, douching, or specimen collection variables have not been determined.
CAUTION: This product requires the handling of human specimens. It is recommended that all human sourced materials be considered potentially infectious and handled with appropriate biosafety practices.
Limitations of the Procedure
- Optimal performance of this kit requires appropriate specimen collection, handling, preparation, and storage.
- This kit should only be used to collect swab samples from the cervix, the vagina, the male urethra, or urine specimens from males and females for testing with the Abbott RealTime CT/NG assay. Other uses of this kit have not been validated.
- The performance of Urine and Swab specimens has not been evaluated in men or women less than 18 years of age.
- The collection of samples from pregnant women using the multi-Collect Specimen Collection Kit should be under the guidance of an obstetrical provider or family physician.
- Vaginal swab sampling is not designed to replace cervical exams for diagnosis of female urogenital infections. Patients may have cervicitis, urethritis, urinary tract infections, or vaginal infections due to other causes or concurrent infections with other agents.
- Women who have symptoms suggesting pelvic inflammatory disease (PID) should not use the self-collected vaginal swab specimen as a replacement for a pelvic exam.
- The self-collected vaginal swab specimen application is limited to health care facilities where support and counseling is available to explain the procedures and precautions.